Abstract
Introduction
Congenital TTP (cTTP) is an ultra-rare disorder in which deficiency of a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13) results in circulating ultra large Von Willebrand Factor (VWF) multimers and subsequent microthrombi formation. Regular prophylactic therapy aims to improve outcomes from long-term complications, but also ongoing symptoms, including lethargy, headaches and abdominal pain, despite normal blood counts. Existing methods of quantifying ADAMTS13 activity lack the sensitivity to enable their use for evaluating treatment response in patients with cTTP. We present a novel flow-based assay with the aim of assessing treatment response, novel therapeutic options and analyzing the impact of different mutations on disease severity.
Method
A VenaFlux semi-automated microfluidic system was used to provide shear flow to mimic in vivo flow rates. Using whole blood, we analyzed platelet adhesion, aggregation and thrombi formation on microchannels coated with type I collagen and mounted onto the stage of an inverted epifluorescence microscope. Fresh, citrated whole blood was treated with DiOC6 to achieve platelet fluorescence and a macro on Image-Pro Premier was designed for automated calculation of total surface coverage. Surface coverage represented increasing thrombus formation with total coverage by thrombus within 180 seconds quantified as 100% coverage.
Results were compared to a normal range developed using 43 normal controls (26=female, 17=male) with normal hemoglobin, platelet count and hematocrit. The surface coverage normal range was 6-39%.
cTTP samples were analyzed for complete blood count, ADAMTS13 activity, VWF antigen, VWF activity and percentage surface coverage. Samples were taken 30 minutes before and after prophylactic treatment, either plasma infusion or BPL-8Y. Recombinant ADAMTS13 was added in-vitro on all pre-treatment samples with 15 minutes incubation time. Further re-measurement was undertaken after initiation of aspirin for at least ten days.
Results
Eighteen patients with cTTP confirmed by genetic analysis and ADAMTS13 levels <5 IU/dl were included (16 = female, 2 = male) with a median age of 33 (range: 15-69 years). Median VWF antigen levels: 114% (range: 54% - 276%, NR: 50-160%) and median VWF activity levels: 173% (range: 83% - 338%, NR: 50-187%).
The median pre-treatment surface coverage was 90% (range 47% - 100%). There was no significant difference in surface coverage considering genetic mutation type (median coverage for homozygous patients 88%, heterozygous 67%, p=0.99), mutation location (pre-spacer mutation surface coverage 67%, post spacer mutation surface coverage 84%, p=0.84), or age of first symptom onset (childhood onset surface coverage 59%, adult onset 86%, p=0.19).
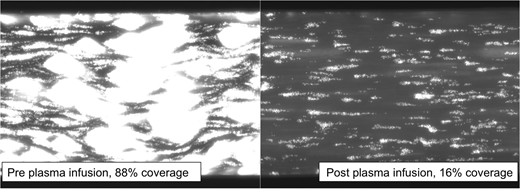
Plasma infusion improved surface coverage results with pre treatment coverage of 90% compared to 44% post plasma infusion (p=0.0003). In vivo recombinant ADAMTS13 administration on pre prophylaxis samples, resulted in normalization of surface coverage in all patients (p<0.0001)(median post rADAMTS13 coverage 28%, range 3-39%). In patients initiated on aspirin, surface coverage had improved both pre and post prophylaxis. The median pre treatment surface coverage for patients on aspirin was 51% (vs. 90% pre treatment and no aspirin, p=0.004). This improvement persisted after treatment with post treatment surface coverage of 18% (vs. 44% post treatment but not on aspirin, p=0.003). 100% of patients who received aspirin saw surface coverage return to the normal range post treatment compared to 82% with plasma infusion alone (p=0.0195).
Conclusion
Plasma infusion and aspirin synergistically reduce surface coverage by thrombus in patients with cTTP, demonstrated on peak and trough samples. Furthermore, in vitro addition of recombinant ADAMTS13 completely normalized thrombus formation. There were no major differences in surface coverage by genetic mutation. The newly developed flow-based assay presented can be used to assess treatment options and efficacy in cTTP in addition to demonstrating cTTP disease pathophysiology that has not previously been identified. In combination with clinical symptoms it offers potential to improve and personalize treatment for patients with cTTP.
Liesner:Bayer: Consultancy, Research Funding; Sobi: Speakers Bureau; Roche: Research Funding; Baxalta: Consultancy, Research Funding; Novo Nordisk: Research Funding, Speakers Bureau; Octapharma: Consultancy, Other: Clinical study investigator for NuProtect Study (Octapharma sponsored), Research Funding, Speakers Bureau. Scully:Novartis: Honoraria, Other: Member of Advisory Board, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal